An Essay on Deliming
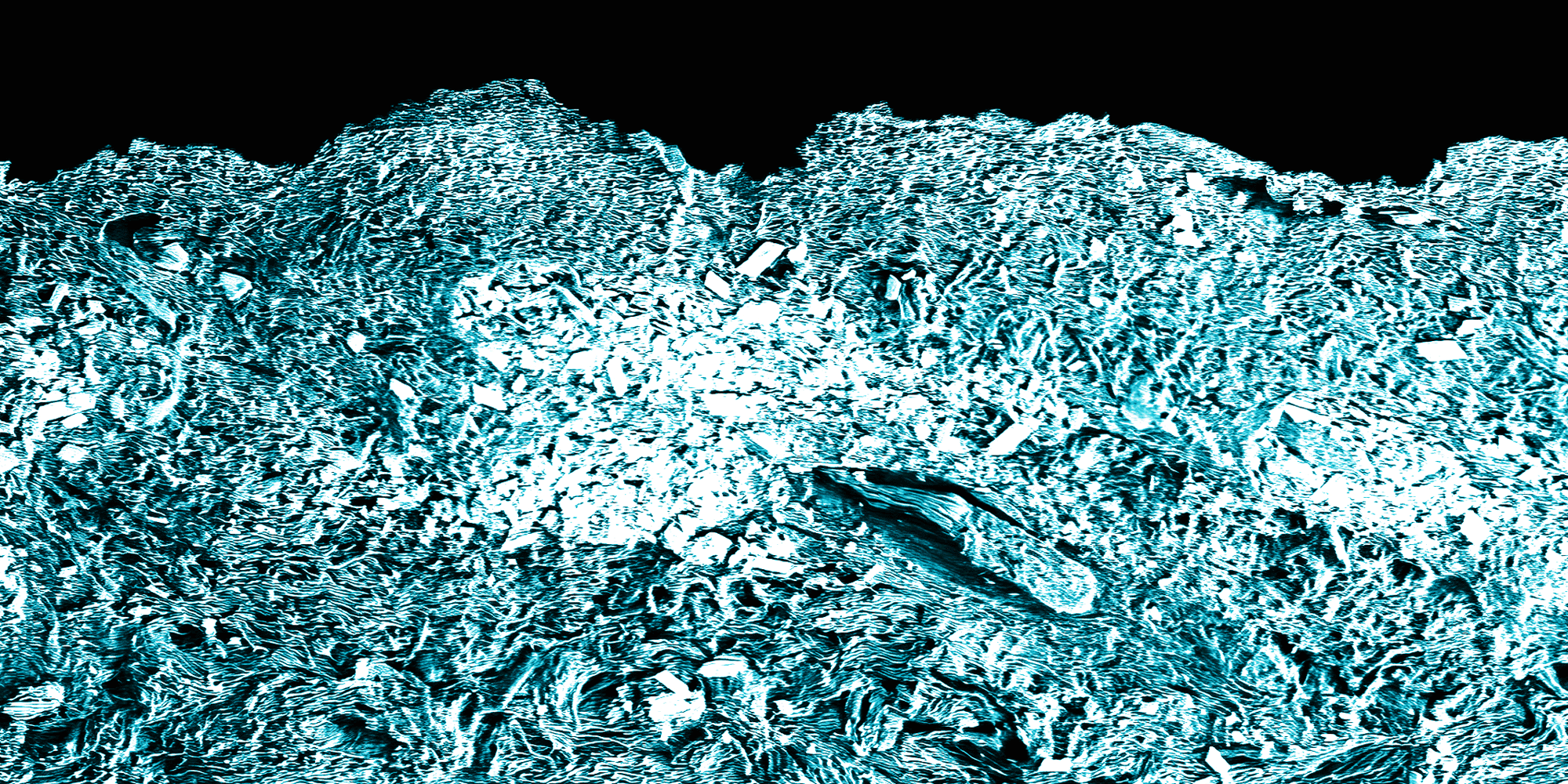
Look how nice these calcium sulfate crystals look up close.

SEM picture of calcium sulfate crystals 1700X
They look nice up close but they’re not so nice when they’re on wet blue. Calcium sulfate crystals form as a result of the reaction between ammonium sulfate and lime. Ammonium sulfate is a popular deliming agent due to its cost-effectiveness, availability, efficiency, safety (thanks to its buffering capacity), and the ability to control deliming either superficially or thoroughly.
Ammonium sulfate, a salt derived from a strong acid and a weak base, typically appears as an off-white substance with varying particle sizes and is highly soluble in water. When ammonium sulfate interacts with calcium hydroxide (lime) in the limed pelt, it produces ammonium hydroxide and calcium sulfate. The calcium sulfate, a white solid with very low solubility, can precipitate either within or on the surface of the wet blue.
Here is the reaction:
(NH₄)₂SO₄(aq) + Ca(OH)₂ (aq) → CaSO₄(s) + 2NH₄OH(aq)
We have occasionally observed that full-thickness wet blue can develop light stains during deliming. These stains become more pronounced after retanning, dyeing, and fatliquoring, causing issues in the crust. The stains result from the precipitation of calcium sulfate, even when ammonium sulfate is only partially used in the deliming formulation.
Below are SEM images of the wet blue illustrating the distribution and shape of calcium sulfate.

Calcium sulfate deposition on the surface of the wet blue SEM 33X

Cross section of the wet blue showing the calcium sulfate deposition inside the grain SEM 37X
Some tanneries can achieve effective deliming using only ammonium sulfate. However, it is more common to mix ammonium sulfate with other deliming agents such as ammonium chloride, dicarboxylic acids, organic acids (formic, citric, acetic, etc.), sodium bisulfate, sodium bisulfite, or commercial deliming products available in powder or liquid form. The reaction of these deliming agents with lime generally results in the formation of soluble salts.
Currently there is an increasing need to reduce or eliminate the use of ammonium sulfate (and other nitrogen-based products including amines) because these chemicals increase the total Kjeldahl nitrogen (TKN) in the wastewater. The discharge of nitrogen is regulated in many cities, states and countries, and the discharge limits are always decreasing.
Buckman specializes in deliming processes. Our skilled technicians collaborate with tanneries to optimize procedures and recommend our Busperse deliming agents, ensuring the production of high quality leather.